A research team led by Professor Ouyang Songying from Fujian Normal University (FJNU) has discovered a unique mechanism explaining how NAD+ depletion is activated in infected bacteria to defend against phage infection in the Sir2-HerA anti-phage defense system. This discovery supports the hypothesis that Sir2 alone in the SaSir2-HerA system does not exhibit NADase activity, which is activated upon binding with HerA.
Sir2, a protein family crucial for depleting NAD+ to halt the growth of infected cells, is activated when its promoters interact with HerA. The researchers used Cryo-EM to study the structure of SaSir2-HerA in complex with ADPR, an NAD+ cleavage product. They found that HerA binding causes the α15 helix above the Sir2 enzyme's active site to transform into a loop and bend away from the ADPR. This helix-to-loop structural transition is vital for activating NADase, ensuring access to the active site.
The findings were published in Nature Communications under the title "Mechanistic basis for the allosteric activation of NADase activity in the Sir2-HerA antiphage defense system," with FJNU as the primary institution. Professor Ouyang Songying, along with Xiong Xiaoli and He Jun from the Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences, are co-corresponding authors. Associate Researcher Zhen Xiangkai, master's student Liu Zihe, and Dr. Wang Xurong from FJNU's School of Life Sciences, along with Dr. Zhou Biao and Dr. Zhao Heyu from the Guangzhou Institute, are co-first authors. The work was supported by grants from the National Natural Science Foundation of China and the National Key Research and Development Program of China.
This research highlights the essential role of Protein Sir2 in NAD+ depletion, a common strategy in bacterial and archaeal defense against phage infections, such as abortive infection. However, the regulatory mechanism of Sir2 as a NAD+ hydrolase remains unclear.
Read the full paper here:https://www.nature.com/articles/s41467-024-53614-6
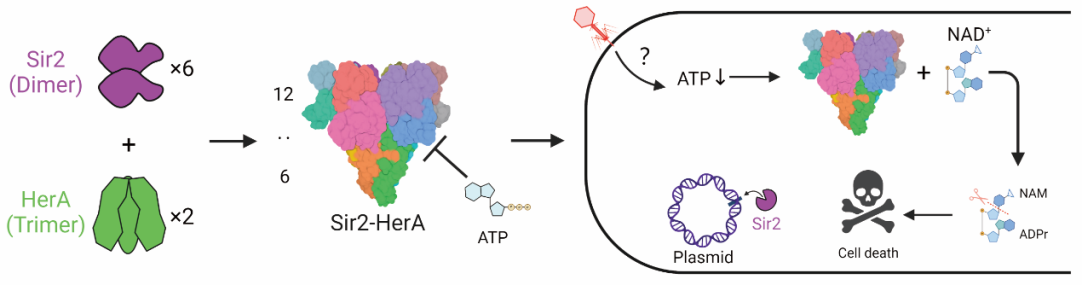
Figure: Summary of the molecular mechanism of the Sir2-HerA anti-phage system.
(Translated by Chen Ziqing, reviewed by Hong Mei)
